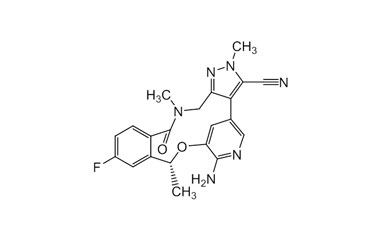
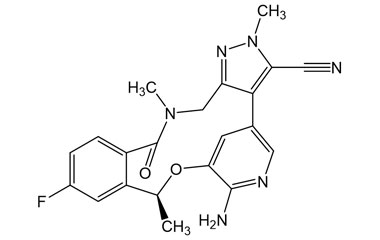
Lorlatinib and its Impurities
Lorlatinib is approved in the US and in Europe for the second- or third-line treatment of ALK-positive metastatic non-small-cell lung cancer (NSCLC). Reference standards of Lorlatinib API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.

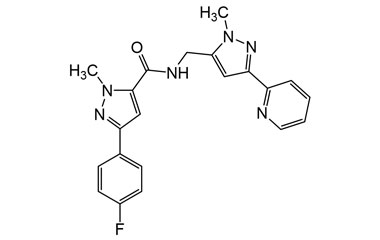
3-(4-Fluorophenyl)-1-methyl-N-((1-methyl-3-(pyridin-2-yl)-1H-pyrazol-5-yl)methyl)-1H-pyrazole-5-carboxamide
3-(4-Fluorophenyl)-1-methyl-N-((1-methyl-3-(pyridin-2-yl)-1H-pyrazol-5-yl)methyl)-1H-pyrazole-5-carboxamide
Catalogue No.:PA 12 1491001
CAS :
2320467-06-7
Molecular Formula : C21H19FN6O
Molecular Weight : 390.42