New Impurity Is Causing the Latest Blood Pressure Medication Recall

Another major recall by FDA.The impurity detected is N-Methylnitrosobutyric acid (NMBA) in Losartan and other sartan drugs. Torrent Pharmaceuticals is recalling lots of losartan-containing products that contain N-Methylnitrosobutyric acid (NMBA) above the acceptable daily intake levels released by the FDA.
Losartan is used to treat hypertension, hypertensive patients with Left Ventricular Hypertrophy and for the treatment of nephropathy in Type 2 diabetic patients. Losartan Potassium and Hydrochlorothiazide tablets, USP is used to treat hypertension and hypertensive patients with Left Ventricular Hypertrophy.
The Food and Drug Administration (FDA) announced,testing has found that NMBA levels in certain lots of losartan potassium blood pressure medications have exceeded the FDA’s interim acceptable intake limits.
Reference Standards of NMBA (N-Methylnitrosobutyric acid) can be easily procured from Pharmaffiliates Analytics and Synthetics Pvt. Ltd.
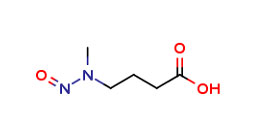
N-Methylnitrosobutyricacid(NMBA)
CAS No - 61445-55-4
Catalog Code - PA 27 04182
For more details : https://www.pharmaffiliates.com/en/pa2704182.html
To obtain more details on this drug recall read : https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/updated-torrent-pharmaceuticals-limited-expands-voluntary-nationwide-recall-losartan-potassium-0






