Meloxicam- An NSAID with a difference

After surgery, post-operative pain is an expected consequence. Opioid analgesics are although used as primary therapy there are many adverse side effects associated with these drugs like nausea, vomiting and respiratory depression as well as acute addiction.
Hence, multimodal strategies for the management of postoperative pain has been recommended by “Practice Guidelines for Acute Pain Management in the Perioperative Setting” adopted by the American Society of Anesthesiology (and many more).
The guidelines state that “unless contraindicated, patients should receive an around-the-clock regimen of Non-steroidal Anti-inflammatory Drugs (NSAIDs), COX-2 inhibitors (COXIBs), or acetaminophen.”
Therefore, to treat acute pain and inflammation post-surgery, Meloxicam which is an enol-carboxamide Non-steroidal Anti-Inflammatory Drug (NSAID) related to Piroxicam, has long been used.
Meloxicam is a preferential cyclooxygenase-2 (COX-2) inhibitor, has long been used to treat osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, as well as various pain syndromes of skeletomuscular origin.
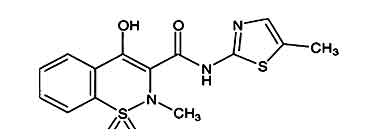
Meloxicam
Meloxicam, in contrast to other NSAIDs, has greater inhibitory activity against the inducible isoform of cyclooxygenase (COX-2) than against the constitutive isoform (COX-1). Meloxicam's anti-inflammatory and analgesic properties are similar to non-selective NSAIDs but it has both gastric mucosal and renal protective properties.
The above discussion proves that Meloxicam as an NSAID has the potential to redefine our expectations of risk-benefit ratio associated with current NSAID treatment of inflammatory disease. This also defines the importance of the use of Meloxicam in the Pharmaceutical parlance.
Drug development and manufacturing of Meloxicam involves the formation of several impurities in the form of degradants, intermediates, and metabolites affecting the efficacy, safety, and purity of the final products.
Identification and isolation of these impurities is a major challenge for the pharmaceutical companies and it is gaining a critical review from the regulatory authorities as well.
Some of the known and unknown impurities of Meloxicam are tabulated below :
| Chemical Name | Product Name | CAS No. | Details |
|---|---|---|---|
| Ethyl 4-Hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxylate 1,1-Dioxide | Meloxicam - Impurity A, Meloxicam USP Related Compound A. | 24683-26-9 | Impurity of Meloxicam and Piroxicam |
| [N(Z)]-N-(3,5-Dimethyl-2(3H)-thiazolylidene)-4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxamide 1,1-Dioxide | Meloxicam - Impurity C | 1262333-25-4 | Impurity of Meloxicam |
| 2-Amino-5-methyl-thiazole | Meloxicam - Impurity B | 7305-71-7 | a major alkaline metabolite of Meloxicam |
| 3-Ethyl-2-imine Meloxicam | Meloxicam - Impurity D | 1331636-17-9 | Impurity of Meloxicam |
| Methyl 4-Hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxylate 1,1-Dioxide | Meloxicam - Impurity E | 35511-15-0 | Impurity of Meloxicam and Piroxicam |
| Methyl 4-Hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxylate 1,1-Dioxide | Meloxicam - Impurity F, Meloxicam USP Related Compound C. | 118854-48-1 | Impurity of Meloxicam and Piroxicam |
| Amido Ethyl Meloxicam | Meloxicam - In house impurity | 881399-30-0 | Impurity of Meloxicam |
| Amido Methyl Meloxicam | Meloxicam - In house impurity | 892395-41-4 | Impurity of Meloxicam |
| 2-(Methylsulfamoyl)benzoic Acid | Meloxicam - In house impurity | 125372-22-7 | Impurity of Meloxicam |
These are some of the potential impurities of Meloxicam which need to be taken care of in the final drug product. ICH guidelines specify the specification limit of the known impurities and the manufacturer has to test these against their reference standards in order to obtain the final product of desired purity.
To obtain a Reference Standard of high quality and purity Pharmaceutical firms need a reliable partner as the quality and integrity of the Reference standard defines the quality of the final product.
The reliable Reference standard provider, provides Pharmaceutical standards, impurities, related substance, and stable isotopes, Reference standards of Meloxicamalong with a comprehensive Certificate of Analysis detailing the characterization process for the material, and ensuring its suitability for both qualitative and quantitative analysis.
The Certificate of Analysis must also take care of all the regulatory requirements.
The dedicated analytical, synthesis and customer service teams at Pharmaffiliates go hand in hand and beyond the standard and in-depth knowledge, decades of manufacturing experience and scientific excellence in the world of reference standards.
Pharmaffiliates Analytics and Synthetics Pvt. Ltd. is a gigantic name as a leading global manufacturer and distributor of reference materials of Pharmaceutical, Phytochemicals, Agrochemicals, Deuterated products, Environmental use.






