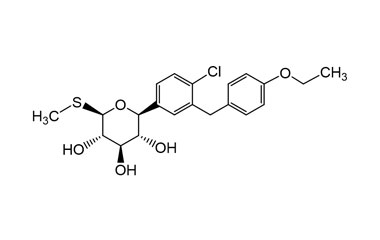
Sotagliflozin
Pharmaceutical standards
- Sotagliflozin has been used in trials studying the treatment of Renal impairment, Hepatic Impairment, Diabetes Mellitus, and High Level of Sugar (Glucose) in the Blood. Reference standards of Sotagliflozin API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
[pname] => Sotagliflozin
[catalogue_number] => PA 19 1610000
[category_ids] => ,82,78,76,75,70,
[chemical_name] =>
[weight] => 424.94
[form] => C21H25ClO5S
[cas] => 1018899-04-1
[pslug] => 1018899-04-1-sotagliflozin-api-pa191610000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA 19 1610000
Molecular Formula : C21H25ClO5S
Molecular Weight : 424.94