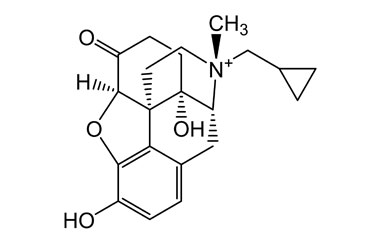
Methylnaltrexone
Pharmaceutical standards
- Methylnaltrexone is approved for the treatment of opioid-induced constipation (OIC). It is generally only to be used when ordinary laxatives have failed. Reference standards of Methylnaltrexone API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
[pname] => Methylnaltrexone
[catalogue_number] => PA 13 2490000
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 356.44
[form] => C21H26NO4+
[cas] => 916055-93-1
[pslug] => 916055-93-1-methylnaltrexone-api-pa132490000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA 13 2490000
Molecular Formula : C21H26NO4+
Molecular Weight : 356.44