vincamine
Impurities
- Vincamine is permitted to be sold as a dietary supplement when labeled for use in adults for six months or less. It is sold in Europe as a prescription medicine for the treatment of primary degenerative and vascular dementia.Reference standards of Vincamine API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
[pname] => cis-Apovincamine-d3
[catalogue_number] => PA STI 008230
[category_ids] => ,69,71,76,75,78,70,82,98,
[chemical_name] =>
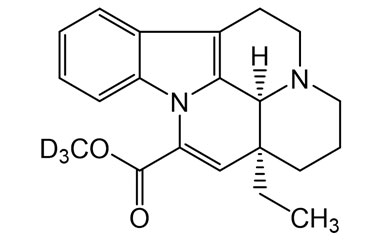
[weight] => 339.45
[form] => C21H21D3N2O2
[cas] => NA
[pslug] => cis-apovincamine-d3-pasti008230
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA STI 008230
Molecular Formula : C21H21D3N2O2
Molecular Weight : 339.45