Lenacapavir
Impurities
- Lenacapavir, sold under the brand name Sunlenca, is an antiretroviral medication used to treat HIV/AIDS. Reference standards of Lenacapavir API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
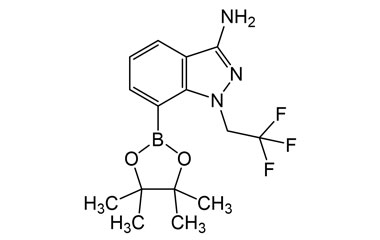
[pname] => 7-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1-(2,2,2-trifluoroethyl)-1H-indazol-3-amine
[catalogue_number] => PA 12 2091002
[category_ids] => ,82,78,76,75,70,
[chemical_name] =>
[weight] => 341.14
[form] => C15H19BF3N3O2
[cas] => 3025145-62-1
[pslug] => 3025145-62-1-7-4-4-5-5-tetramethyl-1-3-2-dioxaborolan-2-yl-1-2-2-2-trifluoroethyl-1h-indazol-3-amine-pa122091002
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
7-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1-(2,2,2-trifluoroethyl)-1H-indazol-3-amine
Catalogue No.:PA 12 2091002
Molecular Formula : C15H19BF3N3O2
Molecular Weight : 341.14
stdClass Object
(
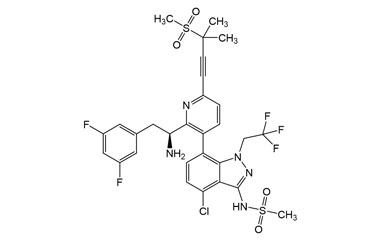
[pname] => (S)-N-(7-(2-(1-Amino-2-(3,5-Difluorophenyl)ethyl)-6-(3-methyl-3-(methylsulfonyl)but-1-yn-1-yl)pyridin-3-yl)-4-chloro-1-(2,2,2-trifluoroethyl)-1H-indazol-3-yl)methanesulfonamide
[catalogue_number] => PA 12 2091015
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 704.13
[form] => C29H27ClF5N5O4S2
[cas] => 3053923-46-6
[pslug] => 3053923-46-6-s-n-7-2-1-amino-2-3-5-difluorophenyl-ethyl-6-3-methyl-3-methylsulfonyl-but-1-yn-1-yl-pyridin-3-yl-4-chloro-1-2-2-2-trifluoroethyl-1h-indazol-3-yl-methanesulfonamide-pa122091015
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(S)-N-(7-(2-(1-Amino-2-(3,5-Difluorophenyl)ethyl)-6-(3-methyl-3-(methylsulfonyl)but-1-yn-1-yl)pyridin-3-yl)-4-chloro-1-(2,2,2-trifluoroethyl)-1H-indazol-3-yl)methanesulfonamide
Catalogue No.:PA 12 2091015
Molecular Formula : C29H27ClF5N5O4S2
Molecular Weight : 704.13