Aripiprazole Monohydrate
Impurities - Aripiprazole Monohydrate was approved by FDA (Abilify trade name) for the treatment of schizophrenia; manic and mixed episodes associated with bipolar I disorder; major depressive disorder; irritability associated with autistic disorder; Tourette's disorder and agitation associated with schizophrenia or bipolar mania. Reference standards of Aripiprazole Monohydrate API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.

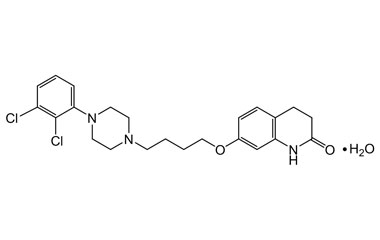
Aripiprazole Monohydrate
Catalogue No.:PA 01 3380000
CAS :
851220-85-4
Molecular Formula : C23H29Cl2N3O3
Molecular Weight : 466.4






