sunitinib
Glucuronides
- An oral anti cancer drug. Works by inhibiting cellular signals by targeting multiple receptor tyrosine kinases (RTKs). Sunitinib is approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) Reference standards of Sunitinib API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
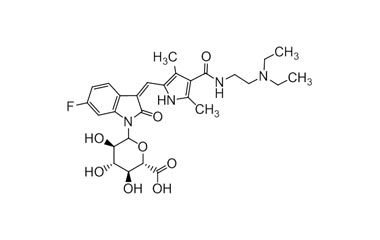
[pname] => Sunitinib N-Glucuronide
[catalogue_number] => PA 19 75670
[category_ids] => ,70,78,82,95,
[chemical_name] =>
[weight] => 574.61
[form] => C28H35FN4O8
[cas] => NA
[pslug] => sunitinib-n-glucuronide-pa1975670
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA 19 75670
Molecular Formula : C28H35FN4O8
Molecular Weight : 574.61