cefamandole nafate
Bases and related reagents
- The clinically used form of cefamandole is the formate ester cefamandole nafate, a prodrug which is administered parenterally. It is no longer available in the United States. Cefamandole Nafate is a second-generation broad-spectrum cephalosporin antibiotic. Reference standards of Cefamandole Nafate API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below
stdClass Object
(
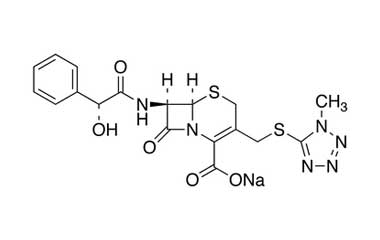
[pname] => Cefamandole Sodium Salt
[catalogue_number] => PA 03 20510
[category_ids] => ,85,83,78,70,82,88,
[chemical_name] =>
[weight] => 484.48
[form] => C18H17N6NaO5S2
[cas] => 30034-03-8
[pslug] => 30034-03-8-cefamandole-sodium-salt-pa0320510
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA 03 20510
Molecular Formula : C18H17N6NaO5S2
Molecular Weight : 484.48
stdClass Object
(
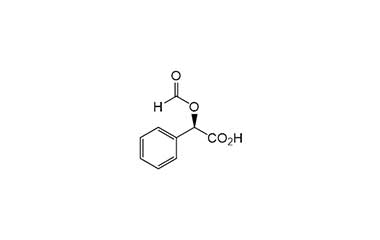
[pname] => (R)-2-(Formyloxy)-2-phenylacetic Acid
[catalogue_number] => PA 03 0201000
[category_ids] => ,85,83,88,78,70,82,
[chemical_name] =>
[weight] => 180.16
[form] => C9H8O4
[cas] => 29169-63-9
[pslug] => 29169-63-9-r-2-formyloxy-2-phenylacetic-acid-pa030201000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(R)-2-(Formyloxy)-2-phenylacetic Acid
Catalogue No.:PA 03 0201000
Molecular Formula : C9H8O4
Molecular Weight : 180.16