zafirlukast
Aromatics
- Zafirlukast is used to prevent asthma symptoms and to decrease the number of asthma attacks in people 5 and older. It is an orally administered leukotriene receptor antagonist. Reference standards of Zafirlukast API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
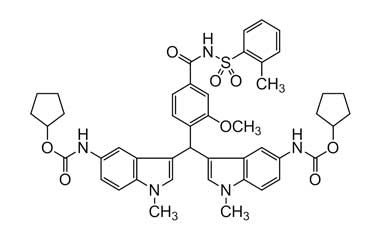
[pname] => Dicyclopentyl (3,3'-((2-methoxy-4-((o-tolylsulfonyl)carbamoyl)phenyl)methylene)bis(1-methyl-1H-indole-5,3-diyl))dicarbamate
[catalogue_number] => PA 26 01540
[category_ids] => ,79,80,71,75,78,70,82,88,
[chemical_name] =>
[weight] => 831.97
[form] => C46H49N5O8S
[cas] => 1160235-24-4
[pslug] => 1160235-24-4-dicyclopentyl-3-3-2-methoxy-4-o-tolylsulfonyl-carbamoyl-phenyl-methylene-bis-1-methyl-1h-indole-5-3-diyl-dicarbamate-pa2601540
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Dicyclopentyl (3,3'-((2-methoxy-4-((o-tolylsulfonyl)carbamoyl)phenyl)methylene)bis(1-methyl-1H-indole-5,3-diyl))dicarbamate
Catalogue No.:PA 26 01540
Molecular Formula : C46H49N5O8S
Molecular Weight : 831.97
stdClass Object
(
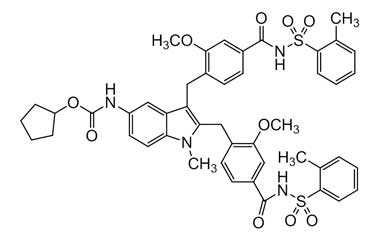
[pname] => Cyclopentyl (2,3-bis(2-Methoxy-4-((o-tolylsulfonyl)carbamoyl)benzyl)-1-methyl-1H-indol-5-yl)carbamate
[catalogue_number] => PA 26 01550
[category_ids] => ,79,80,71,81,76,75,78,70,82,88,
[chemical_name] =>
[weight] => 893.03
[form] => C47H48N4O10S2
[cas] => 1160235-26-6
[pslug] => 1160235-26-6-cyclopentyl-2-3-bis-2-methoxy-4-o-tolylsulfonyl-carbamoyl-benzyl-1-methyl-1h-indol-5-yl-carbamate-pa2601550
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Cyclopentyl (2,3-bis(2-Methoxy-4-((o-tolylsulfonyl)carbamoyl)benzyl)-1-methyl-1H-indol-5-yl)carbamate
Catalogue No.:PA 26 01550
Molecular Formula : C47H48N4O10S2
Molecular Weight : 893.03