levobunolol hydrochloride and its Impurities
Levobunolol Hydrochloride is used topically in the form of eye drops to manage ocular hypertension and open-angle glaucoma. It is a non-selective beta blocker. Reference standards of Levobunolol Hydrochloride API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.

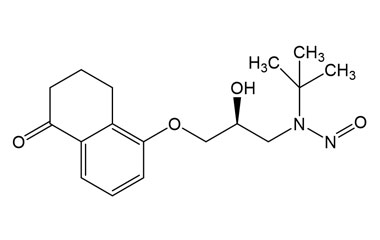
(S)-N-(Tert-Butyl)-N-(2-hydroxy-3-((5-oxo-5,6,7,8-tetrahydronaphthalen-1-yl)oxy)propyl)nitrous amide
(S)-N-(Tert-Butyl)-N-(2-hydroxy-3-((5-oxo-5,6,7,8-tetrahydronaphthalen-1-yl)oxy)propyl)nitrous amide
Catalogue No.:PA 12 0781000
CAS :
NA
Molecular Formula : C17H24N2O4
Molecular Weight : 320.39








