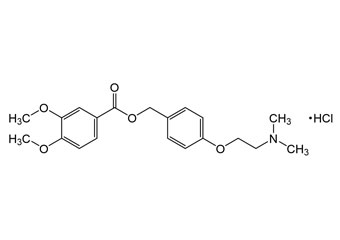
itopride hydrochloride and its Impurities
Itopride Hydrochloride is used for the treatment of Gastroesophageal Reflux Disease, Functional Dyspepsia, and other associated motility disorders. It is a prokinetic benzamide derivative unlike domperidone. Reference standards of Itopride Hydrochloride API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.



![4-[2-(Dimethylamino)ethoxy]benzylamine Dihydrochlo PA 09 29510](https://pharmaffiliates.com/pimages/PA0929510.jpg)
![4-[2-(Dimethylamino)ethoxy]benzylamine PA 09 29520](https://pharmaffiliates.com/pimages/PA0929520.jpg)

![3,4-Dimethoxy-N-[(4-methoxyphenyl)methyl]benzamide PA 09 29540](https://pharmaffiliates.com/pimages/PA0929540.jpg)