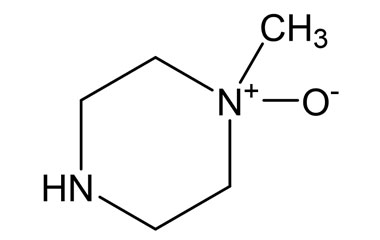
imatinib mesylate and its Impurities
Imatinib Mesylate is used to treat chronic myelogenous leukemia, gastrointestinal stromal tumors and a number of other malignancies. It is sold under the brand names Gleevec. Reference standards of Imatinib Mesylate API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.