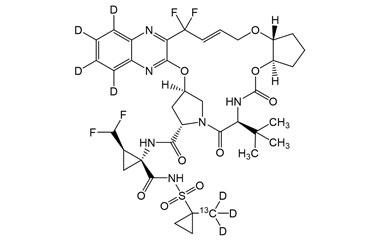
Glecaprevir and its Impurities
Glecaprevir is being developed as a treatment of chronic hepatitis C infection in co-formulation with an HCV NS5A inhibitor pibrentasvir. Reference standards of Glecaprevir API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.


(1R,2R)-1-Amino-2-(difluoromethyl)-N-[(1-methylcyclopropyl)sulfonyl]cyclopropanecarboxamide Hydrochloride
(1R,2R)-1-Amino-2-(difluoromethyl)-N-[(1-methylcyclopropyl)sulfonyl]cyclopropanecarboxamide Hydrochloride
Catalogue No.:PA 07 0851000
CAS :
1360828-80-3
Molecular Formula : C9H15ClF2N2O3S
Molecular Weight : 304.74