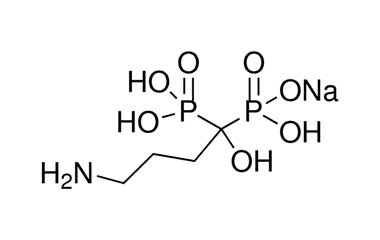
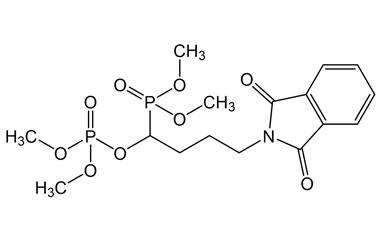
alendronate sodium and its Impurities
Alendronic acid was first described in 1978 and approved for medical use in the United States in 1995. It is available as a generic medication. Reference standards of Alendronate Sodium API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.